 |
| http://www.biology-pages.info/M/mixture.gif |
S&EP - SP7: Engaging in argument from evidence
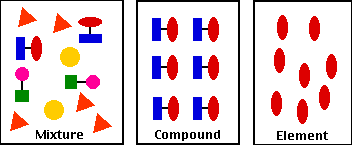
I used evidence to defend my explanation. I answered and filled out a packet of worksheets about elements, compounds, and mixtures, providing the notes that I took in my notebook based off of the slideshow that my teacher put together as evidence. I formulated evidence based on solid data when I stated that elements, compounds, and mixtures are all made up of pure substances, using the fact that elements are pure substances containing only one type of atom, compounds are pure substances containing two or more types of elements that are chemically bonded, and mixtures are when two or more pure substances are together but not chemically combined, as evidence. I examined my own understanding in light of the evidence. I used to think that compounds and mixtures weren't made up of pure substances, but because of the slideshow presentation that my teacher showed my class and I, now I think that compounds and mixtures are definitely made up of two or more pure substances. I collaborated with my peers in searching for the best explanation. I did some research on elements, compounds, and mixtures which I discussed with my class and classmates at my table. Together we figured out that the basic details of the composition of elements, compounds, and mixtures.
XCC: Patterns
As I filled in the packet of worksheets about elements, compounds, and mixtures, I noticed a pattern. I used this pattern to help me fill out a certain activity in the packet, where I had to label if the different listed objects were elements, compounds, mixtures, or none of the above. The pattern that I found to help me identify what pure substance combination each object was, was a pattern in each of the object's formula. For example, the chemical formula of elements is distinctive because it should only contain a single capitol letter. I knew that an object was a mixture when it contained two or more capitol letters in its chemical formula. For instance, I know that a diamond is an element because its formula is C. Even though a diamond element isn't listed on the periodic table, it is made up of only one type of atom which is carbon. As well as I know that something is a compound if its chemical formula contains two or more capitol letters. I know that sugar in particular is a compound because its formula, C6H12O6, contains more than one capitol letter.
As I filled in the packet of worksheets about elements, compounds, and mixtures, I noticed a pattern. I used this pattern to help me fill out a certain activity in the packet, where I had to label if the different listed objects were elements, compounds, mixtures, or none of the above. The pattern that I found to help me identify what pure substance combination each object was, was a pattern in each of the object's formula. For example, the chemical formula of elements is distinctive because it should only contain a single capitol letter. I knew that an object was a mixture when it contained two or more capitol letters in its chemical formula. For instance, I know that a diamond is an element because its formula is C. Even though a diamond element isn't listed on the periodic table, it is made up of only one type of atom which is carbon. As well as I know that something is a compound if its chemical formula contains two or more capitol letters. I know that sugar in particular is a compound because its formula, C6H12O6, contains more than one capitol letter.



